ISO 9001:2015 Quality Management Systems Company
The MEDACX team have a wealth of experience; most have worked within the medical and or drug and alcohol testing industry for over 20 years and for some 30+ years; our team of qualified experienced professionals, are trained and application knowledgeable.
We deliver a quality service and provide professional support to help you meet your requirements through supply of our technology products. We are here to help, you can contact us we are always at the end of a phone to help; be it telephone technical assistance, some product reassurance or if it’s just some ‘on-the-spot’ advice that’s needed simply let us help, good service is key to us.
Training is a key part of what we deliver our team can provide a tailored training program; initial on-site ‘train-the-trainer’ training, web-based training and pdf training guides to suit your organisational needs.
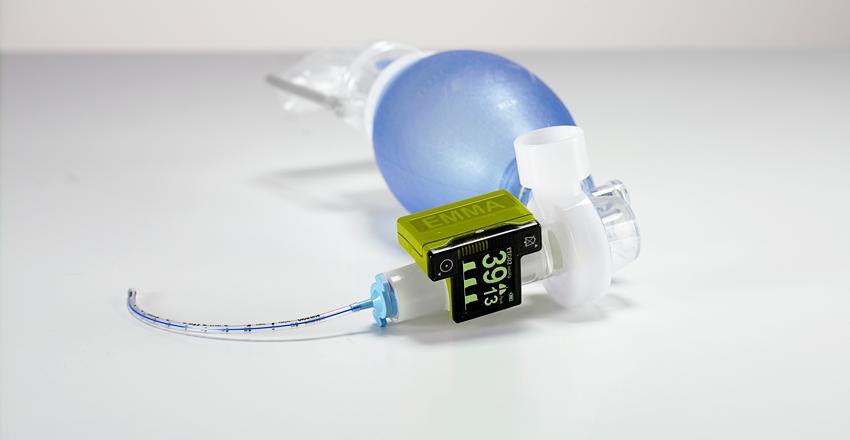
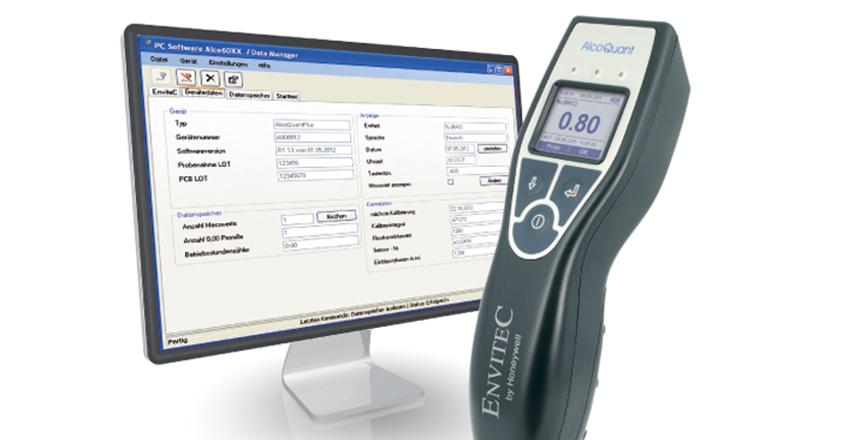
We are an approved supply partner for leading manufacturing companies; Honeywell-EnviteC, Fritz Stephan, Bluepoint-Medical and Masimo. MEDACX is a value added partner providing leading brands; EMMA Waveform®, OxyTrue® SoftCap®, SoftTip®, SoftFlap®, CapnoTrue®, AlcoQuant® 6020, AlcoTrue®, SpiroTrue®, MySign®, VersaStream® and Stephan EVE-TR®, EVE-IN®, EVE_nEO®, EVA® and Sophie®
MEDACX provides quality products and support services and supply to the Home Office, Ministry of Justice, UK Police, Probation service, Prisons, Customs, Counter Terrorism, NHS trusts, Hospitals, Clinics, Surgeries, Basic Doctors, Ambulance teams, Armed Forces Medical teams, Search and Rescue, HART, DAAT’s, Drug Treatment & Rehabilitation, Healthcare, UK Pharmacies, Transport, Maritime industry and Veterinary practices.
Our quality assured technology products and services are providing significant cost savings and improved services.
We utilise the services of a third party UKAS accredited calibration service partner in acordance with ISO17025. Our products are CE marked and meet the relevant standards such as; Medical Device Directives,or MDR and ISO 13485:2016 and fulfil the EC regulations 89/79 EC IVD (in vitro diagnostic products).
MEDACX as part of ISO 9001:2015 is an environmental conscious organisation and we continually improve our environmental, biodiversity and sustainability impact of our activities locally and globally, our company’s commitment is to reduce any impact that our activities might have on the environment and to effectively and efficiently manage resources.
MEDACX is audited annually by the external assessor BSI, a Royal Charter company.
its technology you can trust and designed specifically for the applications and markets we serve..